What the Johnson & Johnson pause is really about.
Why it happened, what it means, and what to expect moving forward.
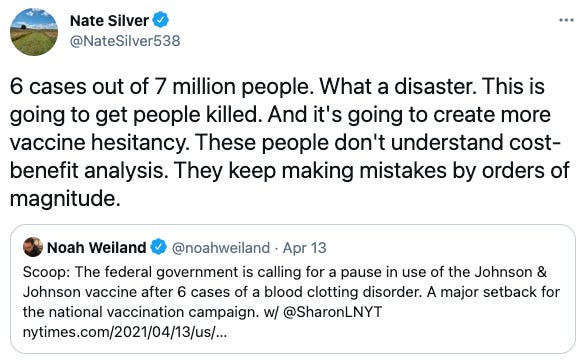
In a startling announcement on Tuesday, a joint statement from the FDA’s Center for Biologics Evaluation and Research and the CDC called for a “pause” on the use of the Johnson & Johnson (J&J) COVID-19 vaccine.
The agencies are investigating the link between the vaccine and a rare form of deadly brain clotting after six recipients—all women between 18 and 48 years old—developed cerebral venous sinus thrombosis (CVST) within thirteen days of receiving the vaccine.
The pause comes in the context of a moment when vaccine hesitancy is eclipsing the lack of vaccine supply as the principal hurdle to achieving herd immunity and ending this pandemic. And this could further shake confidence in the vaccination effort—particularly the one-dose J&J vaccine.
Given that only 6 out nearly 7 million people vaccinated with the J&J vaccine experienced CVST, pundits had a field day dunking on the FDA and CDC for their “mistakes.”
It’s easy to second-guess high-stakes decisions like these from Twitter. What’s harder is putting yourself in regulators’ shoes and trying to play out the long-term consequences of your actions. From there, this pause is far more sensible. Let’s cut in.
The medical ethics at the heart of the pause.
Below is the very first paragraph of the FDA and CDC joint statement calling for the pause. Pay attention in particular to the part I’ve rendered in bold:
As of April 12, more than 6.8 million doses of the Johnson & Johnson (Janssen) vaccine have been administered in the U.S. CDC and FDA are reviewing data involving six reported U.S. cases of a rare and severe type of blood clot in individuals after receiving the J&J vaccine. In these cases, a type of blood clot called cerebral venous sinus thrombosis (CVST) was seen in combination with low levels of blood platelets (thrombocytopenia). All six cases occurred among women between the ages of 18 and 48, and symptoms occurred 6 to 13 days after vaccination. Treatment of this specific type of blood clot is different from the treatment that might typically be administered. Usually, an anticoagulant drug called heparin is used to treat blood clots. In this setting, administration of heparin may be dangerous, and alternative treatments need to be given.
There’s a reason this warning appears in the very first paragraph of the press statement. These regulators were sending a message to healthcare providers, not just the general public.
CVST is exceedingly rare, so it's not usually high on the list of potential diagnoses for people who may be presenting with the non-specific symptoms it causes. If, in fact, CVST is a true side effect of the vaccine, it’s critical that providers ask about whether or not a patient with those symptoms may have received the vaccine in the past three weeks. It radically changes the patient’s management. Though CVST is a clotting event, it should not be treated like other clots—in fact, the usual treatment, a medication called heparin, can make it worse.
While armchair epidemiologists were focused on 6/7,000,000, they were neglecting what that side effect was. This one comes with critical strings attached. Once regulators identified that physicians might not only miss CVST, but make it worse, they had an ethical obligation to alert providers about it.
But how do you alert providers about this without also alerting the public? You can’t—nor should you. That kind of non-transparency would offer endless grist for anti-vax disinformers pedaling conspiracy theories about vaccines.
Getting the science right.
As far as we know, nothing has changed about the J&J vaccine since the pause was announced: it remains safe and effective. The question now is simply for whom. Part of the purpose of the pause is to give science a chance to answer that question.
The first step is to understand whether there truly is a link between the J&J vaccine and CVST or its just a spurious association. To be sure, that question is tricky to answer because the outcome is so rare—both in general and among those who’ve received the vaccine.
If indeed CVST is a true side effect, the next question is in whom it occurs. That gets triangulated somewhere between statistics and biochemistry. Is there something about women between the ages of 18-48 that explains this pathology? There’s plenty of reasons why that might be the case—interactions with the hormones that mediate the ovarian cycle, or pregnancy—for example. But these are conjectures. Scientists will need to dig into the particular pathway that led to the thrombotic thrombocytopenia—or platelet clumping, the biological process mediating CVST—in these six patients.
It also may not be coincidental that this is the second vaccine (the other being the AstraZeneca vaccine, not yet authorized for emergency use by the FDA) that was paused for the same reason. Both share a biological platform, relying on a modified adenovirus to deliver the genetic material that renders immunity. It’s plausible that there may be something about the immune response to the adenovirus vector itself that can activate platelets.
Understanding the mechanism may also help us prevent it. If there’s some set of medications or preparatory regimen that can prevent CVST in people at higher risk, that too, is valuable to understand.
This should reassure people. But it probably won’t.
The system worked! The fact that the safety tracking system built around the deployment of these once-in-a-century vaccines identified six cases of an exceedingly rare event among nearly 7 million recipients of the J&J vaccine should reassure us. It should leave us more secure in the knowledge that if something were to go wrong, they’d know about it. It underlines the safety part of the “safe and effective” vaccines we keep hearing about.
But science doesn’t happen in a bubble. We live in a social media-driven echo chamber that doesn’t tolerate information vacuums and where disinformers lurk in every comment thread to stoke fear. This pause by regulators could inflame vaccine hesitancy and delay—or even prevent—us from achieving the high vaccination rates we need, as some pundits were so quick to point out.
But what if these six cases were ignored? Or if the analyses were hidden—and another person had a CVST or died? The very same pundits would be (rightly) jumping to condemn regulators for failing to have acted sooner. What would the consequences of that have meant for public trust? Preemptive transparency might look foolish in hindsight—but that’s because it prevents us from having to contend with what might have happened had we not acted. It suffers in infamy because it never gets to be compared side by side with the potentially worse reality that it has prevented.
Here’s the kicker: the very same pundits lampooning regulators could be part of the solution if they wanted. Rather than tweet about how wrong and untrustworthy the regulators are to their millions of followers, they could instead be tweeting about how reassuring it is that even the most minute potential side effect was identified and analyzed. They are part of the motive force in a self-fulfilling prophecy.





Any medical treatment should be considered as a risk v. reward potential. Obviously the drug is safe, although one MUST consider the lives of the 6 that did lose their lives, and their loved ones. The part that is highly upsetting is how radicals will attempt to make the sad loss of these six women more relevant than the loss of 550,000 Covid related deaths for the sake of achieving their political talking points.
I cannot finish without noting my proof of this by referring any readers to the abuse of Dr. Fauci by sorry excuses for leaders such as Jim Jordan, Rand Paul, and Ted Cruz. They will grab onto this and exaggerate it until it’s completely distorted into a phony fact only to pump up their disgraceful, Anti-American agenda.
The benefit to risk equation supports the continued use of the J&J vaccine. Recipients could be narrowed by age or sex as needed. Herd immunity must be accomplished world wide to truly control this pandemic. It is now being mentioned that a booster shot may be needed for those who got the Pfizer or Moderna vaccine. We must remained open to change for this novel virus. Time and research will continue to reveal new information.